|
A microchip with an array of 64 nanosensors. The nanosensors appear as small dark dots in an 8 x 8 grid in the center of the illuminated part of the backlit microchip. |
A single centimeter-sized array of the nanosensors can simultaneously and continuously monitor thousands of times more protein-binding events than any existing sensor. The new sensor is also able to detect interactions with greater sensitivity and deliver the results significantly faster than the present "gold standard" method.
"You can fit thousands, even tens of thousands, of different proteins of interest on the same chip and run the protein-binding experiments in one shot," said Shan Wang, a professor of materials science and engineering, and of electrical engineering, who led the research effort.
"In theory, in one test, you could look at a drug's affinity for every protein in the human body," said Richard Gaster, MD/PhD candidate in bioengineering and medicine, who is the first author of a paper describing the research that is in the current issue of Nature Nanotechnology, available online now.
The power of the nanosensor array lies in two advances. First, the use of magnetic nanotags attached to the protein being studied -- such as a medication -- greatly increases the sensitivity of the monitoring.
Second, an analytical model the researchers developed enables them to accurately predict the final outcome of an interaction based on only a few minutes of monitoring data. Current techniques typically monitor no more than four simultaneous interactions and the process can take hours.
"I think their technology has the potential to revolutionize how we do bioassays," said P.J. Utz, associate professor of medicine (immunology and rheumatology) at Stanford University Medical Center, who was not involved in the research.
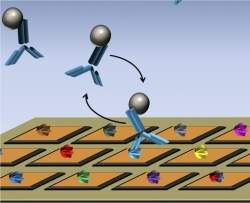
A microchip with a nanosensor array (orange squares) is shown with a different protein (various colors) attached to each sensor. Four proteins of a potential medication (blue Y-shapes), with magnetic nanotags attached (grey spheres), have been added. One medication protein is shown binding with a protein on a nanosensor.
Members of Wang's research group developed the magnetic nanosensor technology several years ago and demonstrated its sensitivity in experiments in which they showed that it could detect a cancer-associated protein biomarker in mouse blood at a thousandth of the concentration that commercially available techniques could detect. That research was described in a 2009 paper in Nature Medicine.
The researchers tailor the nanotags to attach to the particular protein being studied. When a nanotag-equipped protein binds with another protein that is attached to a nanosensor, the magnetic nanotag alters the ambient magnetic field around the nanosensor in a small but distinct way that is sensed by the detector.
"Let's say we are looking at a breast cancer drug," Gaster said. "The goal of the drug is to bind to the target protein on the breast cancer cells as strongly as possible. But we also want to know: How strongly does that drug aberrantly bind to other proteins in the body?"
To determine that, the researchers would put breast cancer proteins on the nanosensor array, along with proteins from the liver, lungs, kidneys and any other kind of tissue that they are concerned about. Then they would add the medication with its magnetic nanotags attached and see which proteins the drug binds with -- and how strongly.
"We can see how strongly the drug binds to breast cancer cells and then also how strongly it binds to any other cells in the human body such as your liver, kidneys and brain," Gaster said. "So we can start to predict the adverse affects to this drug without ever putting it in a human patient."
It is the increased sensitivity to detection that comes with the magnetic nanotags that enables Gaster and Wang to determine not only when a bond forms, but also its strength.
"The rate at which a protein binds and releases, tells how strong the bond is," Gaster said. That can be an important factor with numerous medications.
"I am surprised at the sensitivity they achieved," Utz said. "They are detecting on the order of between 10 and 1,000 molecules and that to me is quite surprising."
The nanosensor is based on the same type of sensor used in computer hard drives, Wang said.
"Because our chip is completely based on existing microelectronics technology and procedures, the number of sensors per area is highly scalable with very little cost," he said.
Although the chips used in the work described in the Nature Nanotechnology paper had a little more than 1,000 sensors per square centimeter, Wang said it should be no problem to put tens of thousands of sensors on the same footprint.
"It can be scaled to over 100,000 sensors per centimeter, without even pushing the technology limits in microelectronics industry," he said.
Wang said he sees a bright future for increasingly powerful nanosensor arrays, as the technology infrastructure for making such nanosensor arrays is in place today.
"The next step is to marry this technology to a specific drug that is under development," Wang said. "That will be the really killer application of this technology."
Other Stanford researchers who participated in the research and are coauthors of the Nature Nanotechnology paper are Liang Xu and Shu-Jen Han, both of whom were graduate students in materials science and engineering at the time the research was done; Robert Wilson, senior scientist in materials science and engineering; and Drew Hall, graduate student in electrical engineering. Other coauthors are Drs. Sebastian Osterfeld and Heng Yu from MagArray Inc. in Sunnyvale. Osterfeld and Yu are former alumni of the Wang Group.
Funding for the research came from the National Cancer Institute, the National Science Foundation, the Defense Advanced Research Projects Agency, the Gates Foundation and National Semiconductor Corporation.